The natural pollution needs help to dissolve in water. Coagulants swoop in to facilitate just that, bridging the gap between undesirable substances and ordinary, clean water.
Coagulants play a critical role in removing impurities. Without chemicals that enter the coagulant level, purification won’t happen, and impurities won’t be removed, which increases the issue of water pollution and makes it unhealthy to consume.
From the water treatment process, we’ll explore the role of coagulants in removing impurities from our water supply.
Table of Contents
- What Coagulants are used in Water Treatment?
- Types of Coagulants Used in Water Treatment
- Coagulation Wastewater Treatment Process
- Advantages of Coagulants in Wastewater Treatment
- Coagulant Selection for Wastewater
- Principal Factors in Coagulant Selection
- Finding the Optimal Coagulant Dosing via Jar Testing
- Optimization Coagulant Dosing
- Evaluation of Coagulant Efficiency and Effectiveness
- Regulation of Coagulant Dosing
- Monitoring and Regulation of Coagulant Feed Rate
- Process Optimization for Maximizing Coagulation Efficiency
What Coagulants are used in Water Treatment?
Before the water in your glass is good enough to suit your taste, a secret army of chemicals –coagulants – ensures you don’t unwittingly invite any unwelcome passengers aboard. Armed with a unique bond-forming power, these essential molecular ties, water-dwelling bugs from sneaking back into the equation.
Types of Coagulants Used in Water Treatment
In the world of water treatment, coagulants are used to neutralize the charges of suspended particles, making it easier to remove them from the water. Two common types of inorganic coagulants are aluminum sulfate, also known as alum, and ferric chloride.
Water purification requires using clever chemicals, and sometimes they can be biodegradable, which has no effect on human health. On the other hand, there are other alternatives, but they are inorganic, like plant- or marine-based coagulants like Zeoturb, starch, cellulose, or tannin derivatives, which are excellent choices for environmentally conscious clarification methods.
The natural pollution needs help to dissolve in water. Coagulants swoop in to facilitate just that, bridging the gap between undesirable substances and ordinary, clean water.
Coagulants play a critical role in removing impurities. Without chemicals that enter at the coagulant level, purification won’t happen, and impurities won’t be removed. This increases the issue of water pollution and makes it unhealthy to consume.
Coagulation Wastewater Treatment Process
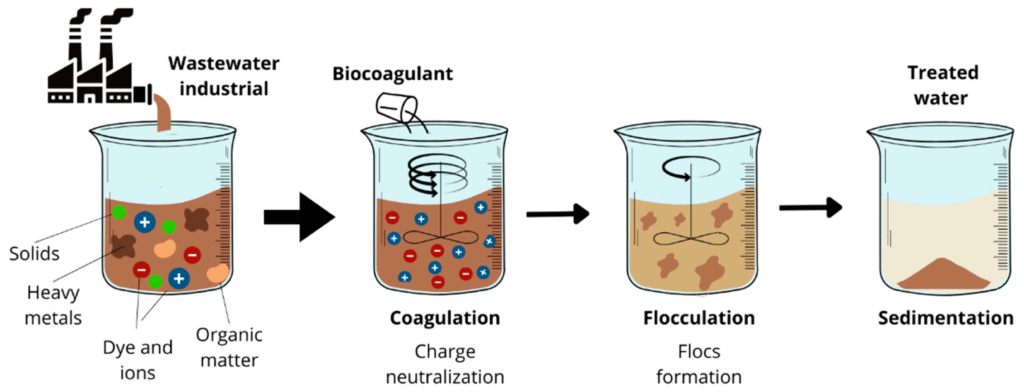
Coagulants destabilize and create large masses known as flocs from small, colloidal impurities during the coagulation water purification process. Coagulants can be easily removed from water by filtration and sedimentation after being created. When mixed with water, coagulants remove the negative charge on suspended material, allowing it to clump together as heavy, more separate masses.
The coagulation water treatment process should ideally occur in two distinct stages:
–Fast mixing—to disperse the coagulant evenly and achieve maximum chemical reaction.
–Slow mixing (flocculation)—to cause particles to collide and allow floc growth.
–Water is then fed to sedimentation tanks after flocculation, where the flocs sink to the bottom and settle to form sludge, which is pumped away for treatment or disposal.
Advantages of Coagulants in Wastewater Treatment
Coagulants are employed in municipal water supply systems and wastewater treatment for industry. Water clarifies, increases disinfection efficiency, and overall water safety by removing turbidity, color, and other pollutants most efficiently.
Apart from the removal of suspended solids, coagulants eliminate natural organic matter (NOM), thereby preventing the formation of toxic disinfection byproducts. During wastewater treatment, coagulation is equally critical in reducing recalcitrant compounds such as phosphorus and fine particulate contaminants that would otherwise persist even when common treatment processes are applied.
Lastly, coagulant application transforms raw water into a clean, safe, and reliable resource to build the foundation of compliant and effective water treatment processes.
Coagulant Selection for Wastewater
Your success with coagulation water treatment will depend heavily on selecting the optimal coagulant. With such a wide variety, making a logical decision requires a step-by-step selection process governed by water quality, treatment objectives, and regulatory requirements.
Principal Factors in Coagulant Selection
Your first step in the direction of coagulant selection is identifying raw water parameters. Key parameters are:
- Turbidity
- pH and alkalinity
- Availability of natural organic matter (NOM)
- Trace heavy metals and other contaminants
Your treatment aims should decide your selection. For example, if the primary application is turbidity control, a particular coagulant may be optimum, but NOM treatment, phosphorus removal, or some other particular pollutant may require another.
Compliance with regulations is likewise not an option. Whether the treated water is to be released or for drinking, there are to be strict safety and quality standards for the coagulant. Failure to do so will be a promise of problem performance, regulatory infractions, and operational costs.
Finding the Optimal Coagulant Dosing via Jar Testing
Following the shortlisting of the potential coagulants, the second step is determining the optimum coagulant dosing for your system through jar testing. It is a test that is conducted in a lab where the shortlisted coagulant is added to the samples of water in varying amounts, and then floc formation, development, and settlement are quantified.
Results give the exact amount of dosage to obtain the desired water quality with the least use of chemicals. coagulant dose calculation enables operators to enhance dosing strategies, maximize process efficiency, and control operating expenses.
Optimization Coagulant Dosing

Optimization Coagulant dosing for effective and uniform coagulant dosing requires complete awareness of process conditions and environmental conditions, i.e., pH, temperature, and raw water conditions, on performance. Proportional adjustment to the optimal coagulant dose is required to provide maximum treatment effectiveness, cost-effectiveness, and regulatory compliance.
Evaluation of Coagulant Efficiency & Effectiveness
Once a coagulant has been selected and the optimal coagulant dosage determined through jar testing, regular evaluation must be conducted to ensure consistent performance at full scale. Performance criteria—such as turbidity removal, floc formation quality, and settling velocities—should be carefully monitored to assure effective treatment.
Monitoring on a regular basis allows for early detection of changes in raw water quality or process conditions. This allows for timely dosing with coagulant or adjusting settling time, preventing potential treatment inefficiencies, compliance issues, and downtime. Active monitoring ensures that water treatment plants always supply safe, high-quality output.
Regulation of Coagulant Dosing
Adjustment of the optimal coagulant dose is a function of multiple interacting factors:
- Raw water quality: turbidity, organics, and seasonality can have a marked influence upon dosage rates.
- Treated water quality specifications: the extent of clarity required and the need for removal of impurities dictate coagulant requirements.
- Downstream treatment processes: needs for filtration and disinfection will influence policy on dosing.
The optimum treatment process will have an optimum combination of these. An optimum combination not only ensures compliance but also optimizes operational efficiency and chemical utilization.
Monitoring and Regulation of Coagulant Feed Rate
Raw water quality varies within hours, and hence, continuous monitoring to regulate Coagulant dosing is a prerequisite. Feed rate adjustment in real time should be done to provide effective treatment.
Water treatment plants that invest in ongoing quality control of turbidity, pH, and other applicable monitored parameters are likely to proceed with change and circumvent under- and over-dosing.
In practice, this results in
- Continued monitoring of performance through jar testing or computer-monitoring systems.
- Quick response rate correction of dosage rates according to real-time water quality data.
- Professional interpretation for the most effective chemical application for maximum effect.
Process Optimization for Maximizing Coagulation Efficiency
There is far more to maximum coagulation efficiency than chemical control process design, and operating precision is just as important.
The best optimizing coagulant dosing methods are:
- Rapid mixing to achieve homogeneous dispersion of the coagulant and complete chemical reaction.
- Adequate optimization of the flocculation process for maximum successful floc-floc and particle-particle collision.
- Regulation of parameters for sedimentation and filtration for maximizing the removal of flocs.
By maximizing each stage of water treatment via coagulation, operators are ideally suited to maximize the capability of their chosen coagulant and provide the best-quality treatment results consistently.
Conclusion
The role of coagulants in water treatment extends far beyond simple impurity removal—they are deliberate enablers of water safety, regulatory compliance, and operational performance. From selecting the right coagulant for raw water conditions to process optimization via jar testing and continuous monitoring, every step of the process directly impacts treatment results and cost-effectiveness.



